Projects
Correlated Electron Materials
Novel Magnetism in Nanoengineered Materials
Professor Deepak Singh at the University of Missouri is leading a project that uses a variety of neutron scattering techniques to study the magnetic behavior of an artificial honeycomb lattice system. The honeycomb lattice structure has generated significant research interest recently—primarily motivated by its unusual electronic and magnetic properties as found in graphene and artificial magnetic honeycomb lattices, respectively. The latter are expected to exhibit a broad and tunable range of novel magnetic phenomena as a function of temperature and magnetic field that are difficult to realize in bulk magnetic materials such as a long-range spin ice, a spin liquid, an entropy-driven magnetic charge-ordered state involving chiral vortex pairs, and spin-order due to the spin chirality. However, it has not been possible to study these phenomena with samples made by current nanofabrication methods (electron-beam lithography), which yield small samples (typical lateral size of ~500 μm2) with large elemental size (connecting wire or bond of the honeycomb lattice). The large typical dimension of individual elements (or bonds) leads to an effective moment of ~107 Bohr magnetons (for permalloy) with an equivalent energy scale of 104-105 K. Therefore, the experimental system is well into its ordered state at any reasonable measurement temperature and thermal fluctuations cannot induce spin flips or the development of a new phase. Also, small sample size rules out the application of powerful probes of condensed matter such as magnetic, heat capacity, and neutron scattering methods that are key to the exploration of novel magnetic phenomena in artificial honeycomb lattices.
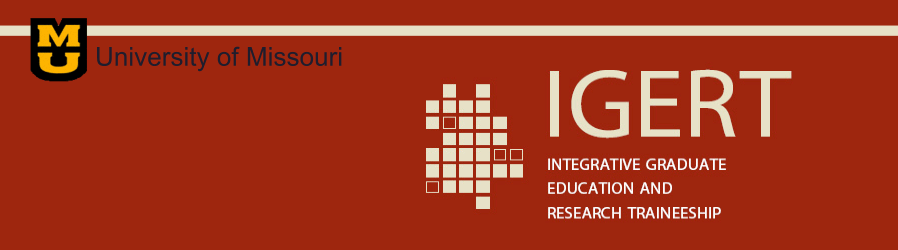
For the first time, Prof. Singh and IGERT trainee Harrison Knoll have been able to create large macroscopic samples of an artificial honeycomb lattice system of connected ultra-small elements (bonds), having length scales of sub-10 nm to 30 nm. The macroscopic size of the sample permits previously impossible experimental studies, including magnetic and neutron scattering measurements. The equivalent energy of the resulting system is 10-100 K (depending on the bond length scale and the magnetic material). Thus these samples are suitable for both temperature and field-dependent exploration of novel magnetic phenomena where the information obtained from a targeted study of related bulk materials can be used as guide. Recently, they performed a small-angle neutron scattering experiment on a 1-in2 cobalt honeycomb sample at the 30-m SANS instrument at the NIST Center for Neutron Research. As shown below in Fig. 1, neutron scattering measurements reveal the development of short-range magnetic order as a function of temperature; thus exhibiting the temperature dependence of the underlying magnetism for the first time in this two-dimensional system.
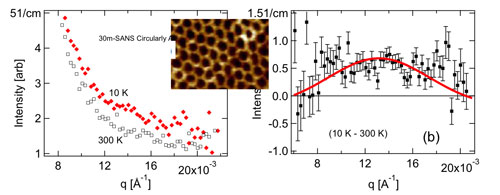
Figure 1: SANS data from an artificial honeycomb lattice system (AFM image shown in the inset): (a) at two different temperatures; and (b) net neutron intensity at 10 K after subtracting high-temperature data.
In other research that Prof. Singh is pursuing within the ambit of our NSF IGERT program, he and IGERT trainee Brock Summers are exploring spin (magnetic) caloritronics phenomena in topographical nanoengineered magnetic materials. Magnetic caloritronics is a newly discovered research arena, which is concerned with spin, charge, entropy and energy transport in magnetic devices. The collective nature of the effects depends on the coupling between spin wave, phonon propagation, and electrons near the sample’s Fermi surface. In their recent research, they have used in-situ nanoengineering to develop a new material, which consists of a locally hexagonal periodic array of ultra-small cobalt dots in direct multidirectional contact with an encapsulating polycrystalline thin copper film. A very unusual behavior is manifested by these new materials in magnetoresistance oscillations that only occur along one magnetic field scan direction in an unusual temperature range of 100 K < T < 200 K. Their preliminary investigations show that the spin-orbit-type coupling between large magnetic moments (formed due to RKKY-type interactions) at localized magnetic sites and a sample’s conduction electrons plays a key role in the observed behavior. Large magnetic moments localized in hexagonal periodic array form a spin-wave spectrum, which arguably couples to the underlying lattice and associated phonons in this system. Detailed neutron scattering measurements on these new materials are vital to understanding the coupling between the lattice order and the magnetic order. They are also seeking to understand the effect of the lattice parameter (center-to-center separation between dots) on both the magnetic and electronic properties of the system, which can be helpful in tuning the properties of the system for practical applications. For this purpose, they are pursuing a detailed experimental plan involving small-angle neutron scattering and thorough electrical measurements.
Nanostructured Materials
Adsorbate-Induced Breathing of Nanoporous Carbon
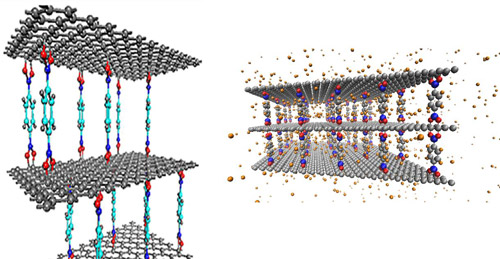
Figure 1: Graphene Oxide Framework structures (left) provide an ideal material for studying adsorbate-induced pore expansion as they can be tuned to various unloaded d-spacings as well as exhibit a Bragg peak. We have performed Molecular Dynamics simulations of the response of the GOF structure to the presence of hydrogen (right). These simulations were also used to calculate the dynamics of hydrogen adsorbed on the material.
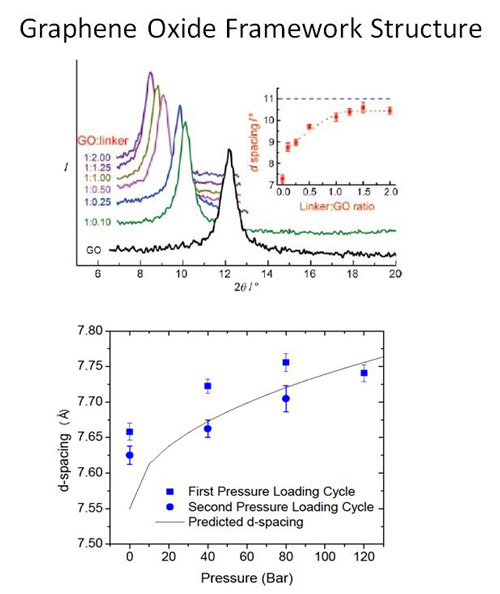
Figure 2: X-ray diffraction measurements of GOF materials synthesized with various linker concentrations show an increase in the separation distance between two graphene oxide layers with increasing linker concentration (top). Neutron diffraction measurements as the GOF is loaded with N2 show an increase in d-spacing as a function of loading pressure (bottom).
An interdisciplinary team of condensed matter theorists and experimentalists, chemists, and neutron scattering instrument scientists led by Professors C. Wexler and J. Burress has developed a combined theoretical, computational, and experimental approach to investigate the dependence of the gas storage capacity of nanoporous carbon adsorbents on pressure. These materials, such as activated carbon and metal organic frameworks, have received significant attention for their potential for storage of hydrogen and natural gas. The team has demonstrated significant structural changes of these nanoporous materials (“breathing”) due to adsorption that affects their gas storage capacity. Their molecular dynamics simulations show the potential for supercritical adsorbed hydrogen to open new pores in a carbonaceous material. Using neutron diffraction, they have demonstrated pore expansion in graphene oxide frameworks (GOFs), a carbonaceous material with tunable slit-shaped pores. GOF materials are composed of graphene oxide layers separated by linker compounds (Figure 1). The separation distance can be tuned during synthesis by varying the linker concentration (Figure 2, top). Our goal is to determine the dependence of the separation distance on species (H2, CH4, N2 and CO2) and loading pressure of adsorbed gas. We have measured a 2% increase in separation distance with N2 adsorbed at room temperature at 120 bar (Figure 2,bottom). We are currently developing a gas manifold to allow for gas loading in situ during diffraction measurements at MURR. We have measured the dynamics of hydrogen in the GOF material at the BASIS instrument at the Spallation Neutron Source at Oak Ridge National Laboratory (ORNL). The Q-dependence of the broadening of the quasi-elastic line suggest an increase in diffusion rates for hydrogen as a function of loading pressure (Figure 3). This type of dependence (an increase, rather than a decrease with increasing pressure found in static structures) has been predicted for breathing materials through Molecular Dynamics simulations.
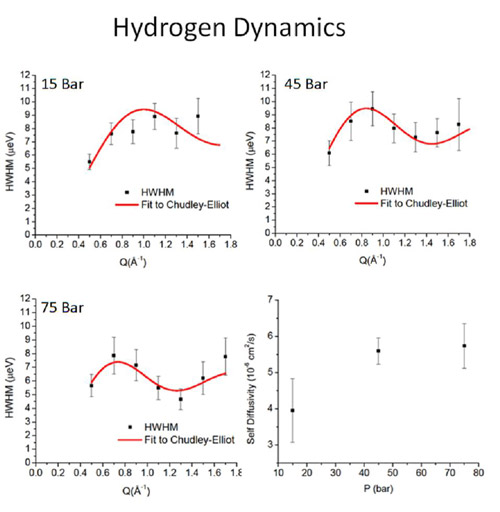
Figure 3: Quasielastic neutron scattering measurements of the dependence of the broadening of the quasi-elastic line with momentum transfer . The measurements show an increase in hydrogen self-diffusivity (bottom right) as a function of pressure consistent with molecular dynamics simulations of breathing materials.

Figure 4: Matthew Connolly uses a gas-loading manifold at the BASIS instrument at ORNL to load the GOF sample with hydrogen up to 200 bar.
Oscillating Heat Pipes: Imaging in Operation with Neutrons
Hongbin Ma (Mechanical and Aerospace Engineering), Haskell Taub (Physics), and Robert Winholtz (Mechanical and Aerospace Engineering) of the University of Missouri have found methods to improve the performance of oscillating heat pipes (OHPs) for cooling applications, particularly cooling electronics. Using neutrons, the NSF-funded Integrative Graduate Education and Research Traineeship team has also gained a better understanding of the complex mechanisms at work in effectively transferring heat in an OHP [1].
Oscillating heat pipes are a promising new heat removal technology that show great promise for cooling electronics which is becoming a problem with increased miniaturization and power demands. An OHP consists of a channel that travels back and forth between an evaporator section that removes heat from a heat source and a condenser section that rejects heat to a cold source. An OHP created in a flat plate of copper is illustrated in Fig. 1. The channel is evacuated and partially backfilled with a working fluid such a water. The channels must be small enough to develop alternating regions of liquid and vapor slugs. When heat is input the liquid vaporizes increasing pressure near the evaporator and pushing the liquid slugs around the channels where they transfer heat to the condenser where the vapor can condense reducing the pressure.
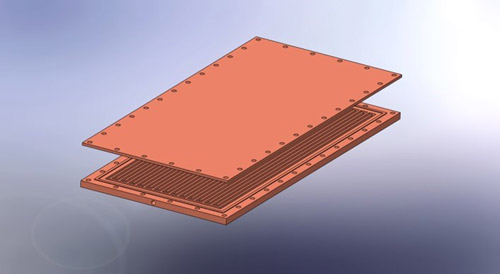
Fig. 1. Illustration of a flat flate oscillating heat pipe. A copper plate has a single channel milled in it that moves back and forth between ends of the plate and is sealed with a copper sheet. Heat is transported from one end, the evaporator, to the other, the condenser, with a liquid/vapor mixture in the channel. Pressure differences between the ends due to evaporation and condensation drive the fluid flow within the channel.
It has been found that hydrophilic nanostructured surfaces inside the OHP channels can enhance the heat transfer performance. Furthering this line of work, an OHP was produced with only half of the area made hydrophilic. This half could be made the evaporator or the condenser by switching which end heat was input on and which end heat was removed from. A hydrophilic cupric-oxide nanostructured surface was created on half the OHP using the procedure outlined in Liu et al. [2]. A scanning electron microscope image of this nanostructured surface is shown in Fig. 2. The thermal performance of the OHP was investigated as shown in Fig. 3. The entire setup was placed in the Neutron Imaging Facility at the NIST Center for Neutron Research so that neutron video of the working fluid could be simultaneously acquired.
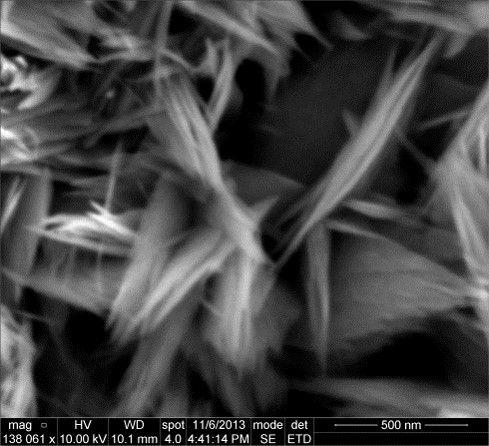
Fig. 2. Nanostructured cupric oxide surface produced on the interior channels of the OHP to enhance its thermal performance.
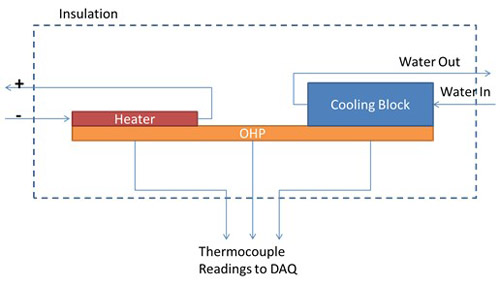
Fig. 3. Experimental configuration to test the thermal performance of the OHP. Heat is input with a resistive heater on one end of the OHP, the evaporator. On the other end of the OHP the condenser, an aluminum block with temperature-controlled water running through interior channels, removes the heat. Thermocouples record the temperature on the evaporator and condenser ends of the OHP and the entire setup is surrounded by fiberglass insulation to prevent any other heat transfer. The thermal performance is given by the difference in temperature between the evaporator and condenser. A smaller temperature difference indicates a better thermal performance.
The thermal performance, measured as the difference between the evaporator and condenser, was measured as a function of heat input for three different OHP configurations (1) an OHP with no treatment to the channel surfaces but otherwise identical, (2) an OHP with hydrophilic nanostructured cupric-oxide on one half configured with the nanostructured half as the evaporator, and (3) the same OHP but flipped around so that the nanostructured surface half was connected to the cooling block making it the condenser. The thermal performance results are shown in Fig. 4. Heat input to the evaporator was gradually increased and temperatures recored while neutron video was also simultaneously acquired. The untreated OHP showed linearly increasing temperature difference initially, similar to a solid material, while the interior fluid was stationary. At about 120 W heat input the interior liquid and vapor began moving and the performance plateaus. The OHP with nanostructures in the evaporator shows similar behavior at low heat input but shows marked improved performance once fluid motion begins. The OHP with the nanostructures in the condenser begins oscillating at a lower heat input and shows the best performance over the range of 70 to 175 W. At 200 W and above its performance matches the untreated OHP.
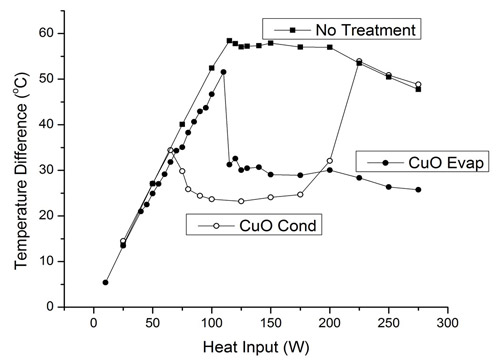
Figure 4. Thermal performance of three OHP configurations as measured by the difference in temperature between the evaporator and condenser. First an OHP with no surface treatment was measured. An OHP with nanostructures on the evaporator half was then investigated followed by reversing the heater and cooling block making the condenser end contain the nanostructured surfaces.
The accompanying neutron video gives some nice insight into the behavior of the OHP with nanostructures in the condenser. Two of the videos can be seen in Figure 5. Between 70 and 175 W the fluid is moving vigorously which is beneficial to the enhanced performance. At higher heat input the liquid is pushed more completely into the condenser region by the increased pressure in the evaporator from the higher heat input. The motion is also less vigorous, similar to the other OHPs. As can be seen in the video at 225 W, when the liquid is in the condenser it covers up the nanostructured surfaces in the condenser most of the time preventing the vapor from contacting the nanostructures. The lack of contact of vapor and the nanostructures can be seen to correspond to the degradation in the performance of the condenser nanostructures OHP seen occurring between 175 and 225 W. Presumably this inhibits condensation on the nanostructures in the condenser and the OHP behaves quite similar in performance to the untreated OHP.
Figure 5. Neutron videos of the OHP with hydrophilic cupric-oxide nanostructures in the condenser region, (a) 75 W heat input and (b) 225 W heat input. At 75 W the fluid motion is vigorous and the vapor phase (lighter) has significant contact with the nanostructured surfaces in the condenser. At 225 W the fluid has been pushed fully into the condenser by the pressure developed in the evaporator and the fluid motion is less vigorous. The liquid phase completely covers the nanostructures preventing the vapor phase from contacting it, presumably inhibiting condensation.
References:
1. F.Z. Zhang, R.A. Winholtz, W.J. Black, M.R. Wilson, H. Taub, and H.B. Ma, “Effect of Hydrophilic Nanostructured Cupric Oxide Surfaces on the Heat Transport Capability of a Flat-Plate Oscillating Heat Pipe,” J. Heat Transfer, 138, 062901-1 (2016).
2. Liu, J., Huang, X., Li, Y., Sulieman, K.M., He, X., and Sun, F., "Hierarchical Nanostructures of Cupric Oxide on a Copper Substrate: Controllable Morphology and Wettability." J. Materials Chemistry, 16, 4427-4434 (2006).
Biomaterials
Neutron Scattering Study of Water Diffusion on Single-Supported Bilayer Lipid Membranes
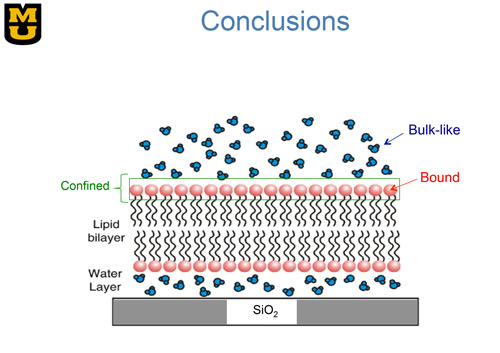
Figure 1. Sketch of a single bilayer lipid membrane supported on a surface of silicon oxide. Each lipid molecule consists of a polar head group (red) connected to a tail consisting of two alkyl chains (black). The associated water molecules are shown in blue. As suggested by Castellana and P. S. Cremer, Surf. Sci. Rep. 61, 429 (2006).
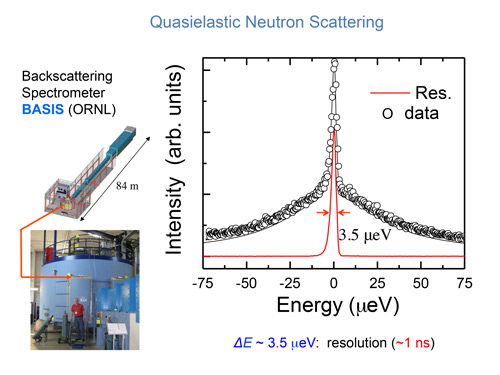
Figure 2. Lower left: The neutron spectrometer BASIS at the Spallation Neutron Source, Oak Ridge National Laboratory, one of the instruments used to study the diffusion of water in samples of model membranes. Determination of the incident neutron energy to high precision requires an 84-meter long flight path as shown above the spectrometer. Right panel: Typical spectrum of neutrons scattered from the water molecules in a single-supported membrane sample. The smallest change in energy of the neutron that the spectrometer can detect is 3.5 micro electron volts. This energy resolution corresponds to a water molecule performing a random translational motion with each step of its motion taking about 1 ns.

Figure 3. IGERT trainee Andrew Miskowiec makes an adjustment on the sample stage of the neutron reflectometer at the University of Missouri Research Reactor.
Profs. Haskell Taub and Flemming Hansen and their team members at the University of Missouri and the Technical University of Denmark have successfully used neutron scattering and computer simulations to characterize the random motion of water molecules associated with a bilayer lipid membrane. Their achievement is significant because bilayer lipid membranes form the boundary of virtually all living cells; and an understanding of the structure and motion of the membrane-associated water is essential for explaining how the membrane functions. Because of its sensitivity to hydrogen atoms, neutron scattering is very effective for probing the motion of membrane-associated water. Unfortunately, due to the weak scattering from a single membrane, previous neutron scattering experiments have used samples consisting of stacks of thousands of membranes supported on a solid substrate. But such experiments are difficult to model in computer simulations. The sheer number of membranes in a multilayer stack, the interactions between membranes, and the presence of unknown amounts of water between membranes complicate the interpretation of the neutron spectra.
Our interdisciplinary team has been able to solve the problem of using stacks of many membranes by depositing a single bilayer membrane onto an oxide-coated silicon substrate as shown in Fig. 1. By stacking a hundred of such wafers, we have obtained an adequate intensity of scattered neutrons and yet retained a sample simple enough for simulation on a computer. Using such a sample, we performed high-energy-resolution neutron scattering experiments at the NIST Center for Neutron Research and the Spallation Neutron Source at Oak Ridge National Laboratory. The neutron scattering spectrometer used in the Oak Ridge measurements is shown in the lower left of Fig. 2. One can appreciate the size of the spectrometer by comparing it with Prof. Hansen standing in front. This size is needed to accommodate the many neutron detectors required to increase the counting rate in the experiment. The long 84-meter flight path shown above the spectrometer enables the energy of the neutrons incident upon a sample to be determined with high precision.
Figure 3 shows IGERT trainee Andrew Miskowiec performing preliminary neutron scattering measurements at the University of Missouri Research Reactor. By analyzing the inelastic neutron spectra from water molecules associated with the single-supported membrane sample, our team was able to infer three different types of water motion as indicated in Fig. 1: 1) bulk-like motion of water situated well above the membrane; 2) motion of water confined to a region near the lipid head groups; and 3) motion of bound water that is hydrogen-bonded to the head groups [1]. The motion of bulk-like and confined water molecules is faster than those bound to the lipid head groups, which move on the same nanosecond time scale as H atoms in the lipid molecules. Our results were found to be consistent with computer simulations on a freestanding bilayer lipid membrane [2].
References:
1. M. Bai, A. Miskowiec, F. Y. Hansen, H. Taub, T. Jenkins, M. Tyagi, S. O. Diallo, E. Mamontov, K. W. Herwig, S.-K. Wang, “Study of the Water Diffusion on Single-supported Bilayer Lipid Membranes by Quasielastic Neutron Scattering,” Europhys. Lett. 98, 48006, 1-6 (2012).
2. F. Y. Hansen, G. H. Peters, H. Taub, and A. Miskowiec, “Diffusion of Water and Selected Atoms in DMPC Lipid Bilayer Membranes,” J. Chem. Phys. 137, 204919, 1-15 (2012).